Discovering and developing novel medicines for acute and chronic degenerative diseases
Cyclophilins are ubiquitous proteins that have important functions both inside cells and as secreted proteins. Acting as peptidyl-prolyl cis/trans isomerases they control shape transitions of proteins. Because the shape of a protein determines its function, cyclophilins and related enzymes exert control over the functions of their client proteins, governing protein-protein interactions in routing the information flow through cellular networks. Their multiple functions include controlling the fate of cells that are stressed by exogenous factors such as trauma, infection, radiation, or toxins, but also by endogenous factors such as excessive signals from physiological stimuli or metabolic dysregulation.
Many viruses rely on cyclophilins supplied by their horst to establish an infection and bacteria have shown to secrete cyclophilins to manipulate the immune response. Emerging data suggest as yet not understood roles in the innate immune system indicating potential roles in the tumour microenvironment.
Inhibitors of cyclophilins have therefore a broad spectrum of potential therapeutic applications where broad physiological damage control is essential. These situations include
acute diseases caused by tissue injuries, chronic degenerative and
fibrotic diseases, virus infections and
oncology.
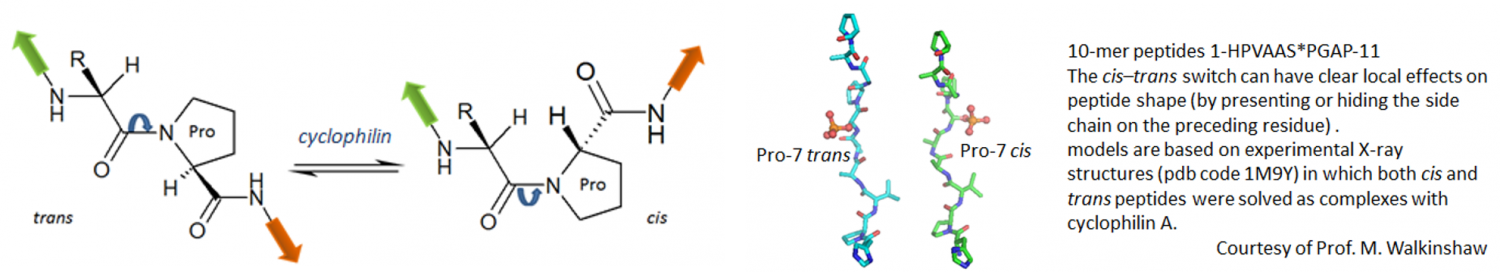
Cyclophilins were initially identified as binding proteins of the immunosuppressant drug cyclosporin (Sandimmun™, Neoral™). However, the existence of cyclosporin analogues (e.g. NIM-811) that retained inhibitory potency to cyclophilin but completely lacked immunosuppression demonstrated (1) the existence of non-immunosuppressive cyclophilin inhibitors, (2) that the biology exhibited by these compounds differed substantially from that of cyclosporin and (3) that cyclophilins are a target class that is amenable to inhibition by small molecules. As the wider role of cyclophilins in disease has become better understood, the therapeutic potential of cyclophilin inhibitors has become clearer. Cyclophilins are used as virulence factors by many viruses (HIV, HCV, coronaviruses, others) and non-immunosuppressive cyclophilin inhibitors have been evaluated in clinical studies for the treatment of infection by HCV and HIV (Alisporivir, SCY-635).
Of all the human cyclophilins, cyclophilins A and D are by far the most intensely investigated proteins. Cyclophilin A is predominantly an intracellular protein but can in certain circumstances be released into circulation where, through interaction with CD147 and other membrane receptors, it acts as a powerful pro-inflammatory mediator 1
. Secretion of cyclophilin A from many cell types is triggered by LPS, ROS, or various forms of necrotic cell death. In fact, circulating cyclophilin A has been proposed as a biomarker for necrotic cell death 2
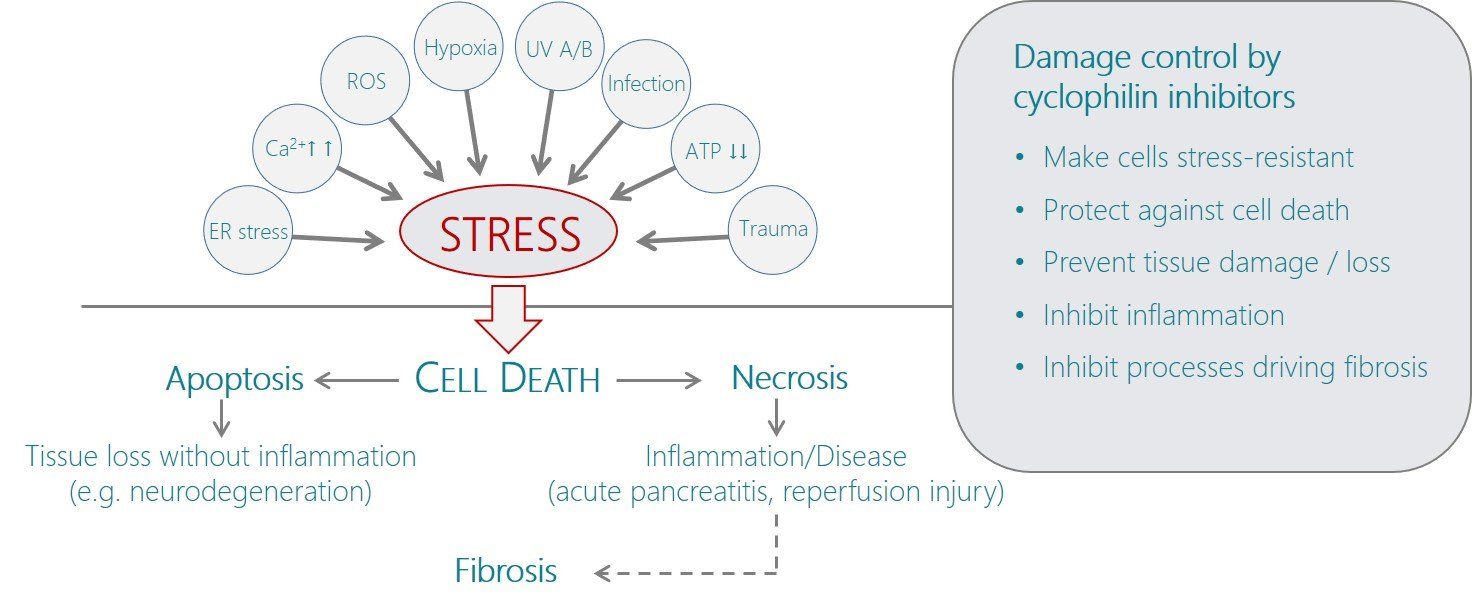
. Cyclophilin D is a component of the mitochondrial permeability transition pore (MPTP) which plays a key role in cell death by regulating the threshold of MPTP opening 3
; absence or pharmacological inhibition of cyclophilin D makes the pore more resistant to opening stimuli, which in the widest sense include any factor that stresses the cell, including toxins, infection, hypoxia, reactive oxygen species (ROS), UV radiation, or Ca 2+
overload (i.e. excessive physiological signals). MPTP-mediated cell death can occur by many mechanisms that can be broadly categorised into apoptotic (non-inflammatory) or necrotic (inflammatory) pathways. Thus, inhibition of cyclophilin D by increasing the threshold to MPTP opening protects cells from both apoptotic and necrotic death and is therefore expected to slow/halt chronic degenerative diseases as well as inflammatory conditions caused by necrotic cell death or infection 4
. Dual inhibitors of cyclophilins A and D can therefore be viewed as damage control agents that provide some protection against stress-induced death of cells as well as the associated inflammatory processes at the same time. Such situations occur e.g. in trauma-induced sepsis, where patients often survive the early period after trauma but suffer morbidity and complications during the subsequent treatment phase, or in acute pancreatitis where diet-induced necrosis of the acinar cells triggers a violent inflammation that in severe cases can lead to acute respiratory distress syndrome and multiple organ failure 4
.
References
- Yurchenko, V. et al. (2010), “Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics”, Clin. Exp. Immunol. 160: 305-17Christofferson & Yuan (2010), “Cyclophilin A release as a biomarker of necrotic cell death”, Cell Death Different. 17(12): 1942-43
- Christofferson & Yuan (2010), “Cyclophilin A release as a biomarker of necrotic cell death”, Cell Death Different. 17(12): 1942-43
- Giorgio V et al. (2010) “Cyclophilin D in mitochondrial pathophysiology”, Biochim. Biophys. Acta 1797(6-7): 1113-18
- Fliri, H. (2016), Drug Target Review, “Neurodegenerative diseases: The potential of cyclophilin inhibition”, Jan 21
- Jin H et al., (2014) “Prediction of sepsis in trauma patients”, Burns Trauma 2(3):106-113
Read the whole paper, Prolyl Isomerases as Therapeutic Targets, co-written by Selcia and Cypralis scientists.

